|
|
|
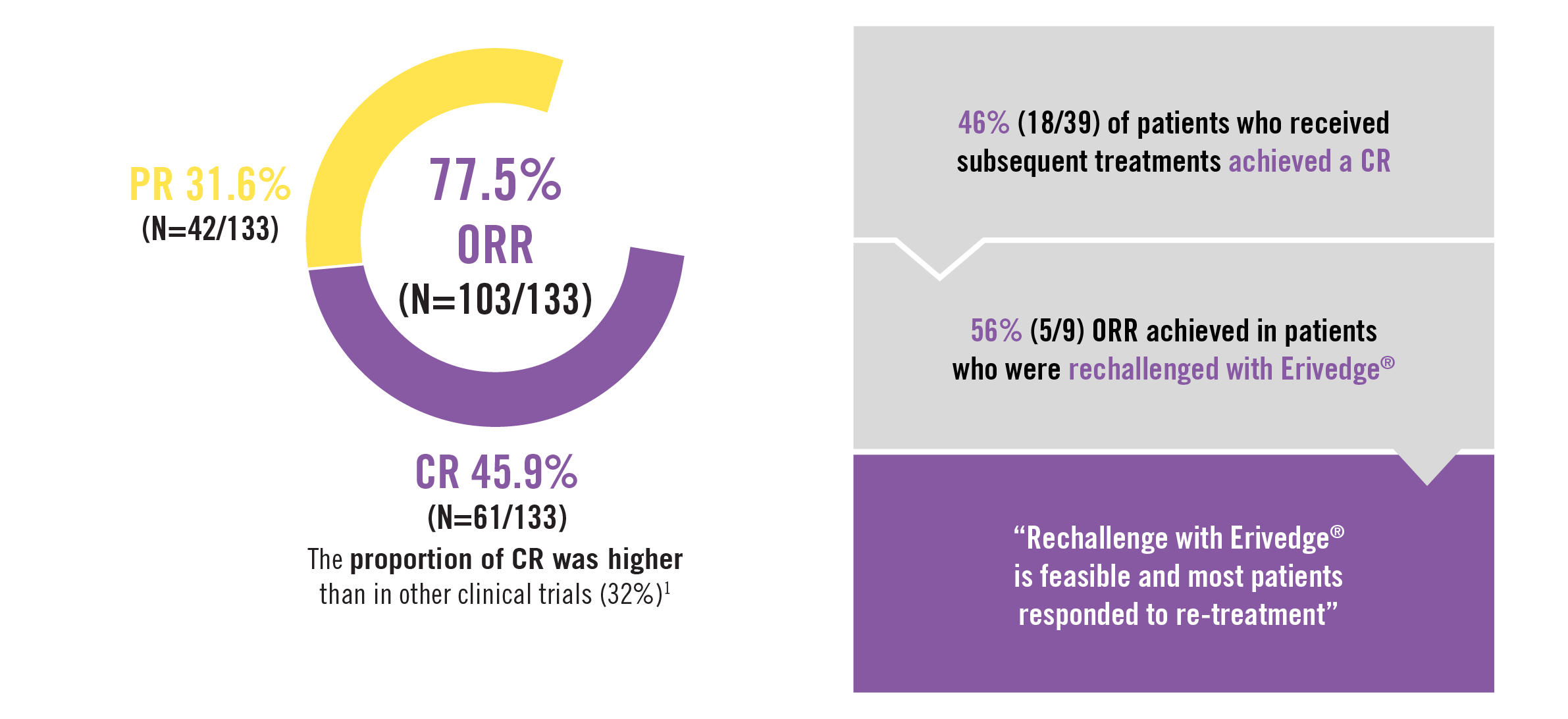
| Efficacy of Erivedge® after a median treatment duration of 32.5 weeks (20.9-56.1)1 |
|
Most frequent reasons for interrupting Erivedge®1 |
|
|
|
Most common grade 3/4 AEs were muscle cramps (8 patients; 6.0%), asthenia (3 patients; 2.3%) and weightloss (2 patients; 1.5%) |
The risk of recurrence after achieving CR with Erivedge® was lower than in previous reports. The RELIVIS study confirms the safety and efficacy of Erivedge® in aBCC1 |
|
Please see full prescribing information for additional important safety information |
|
| M-XX-00018376 |