|
Retrospective, multicentre study highlighting the value of Erivedge® maintenance therapy in aBCC |
|
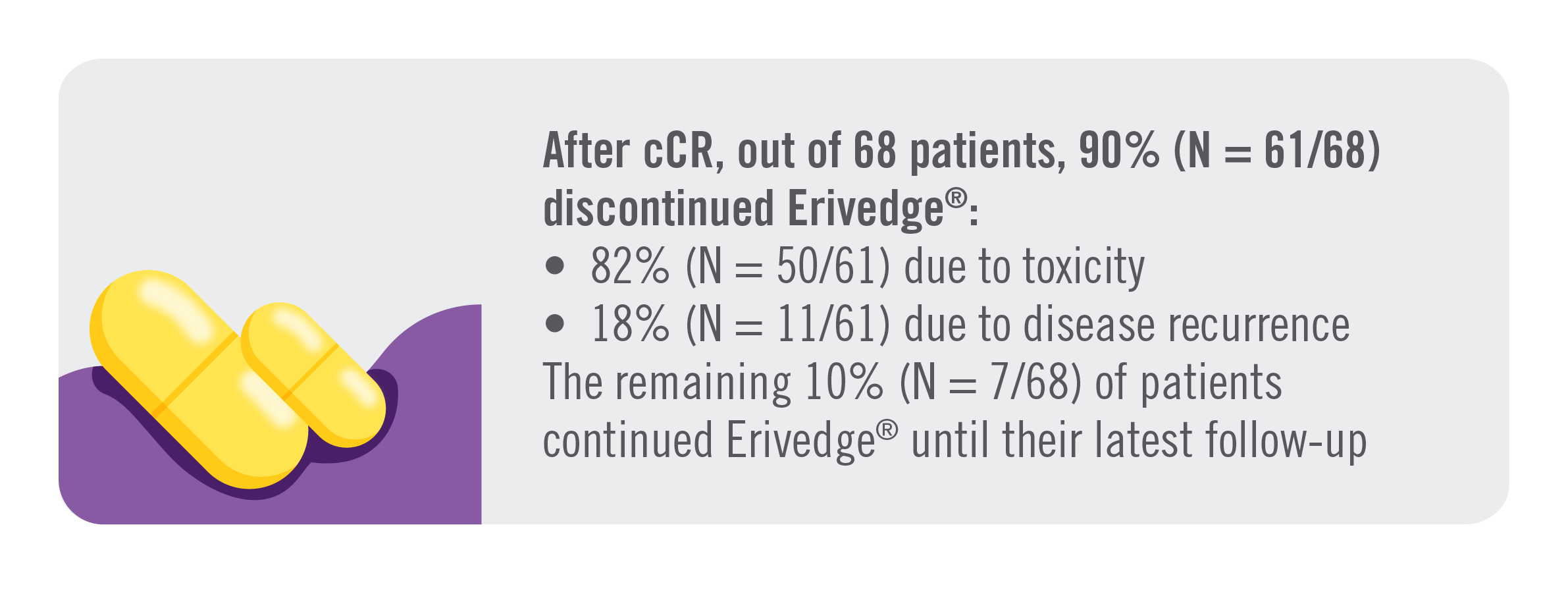
Main outcomes at median follow-up of 42.5 months (range 0–91)1 |
|
|
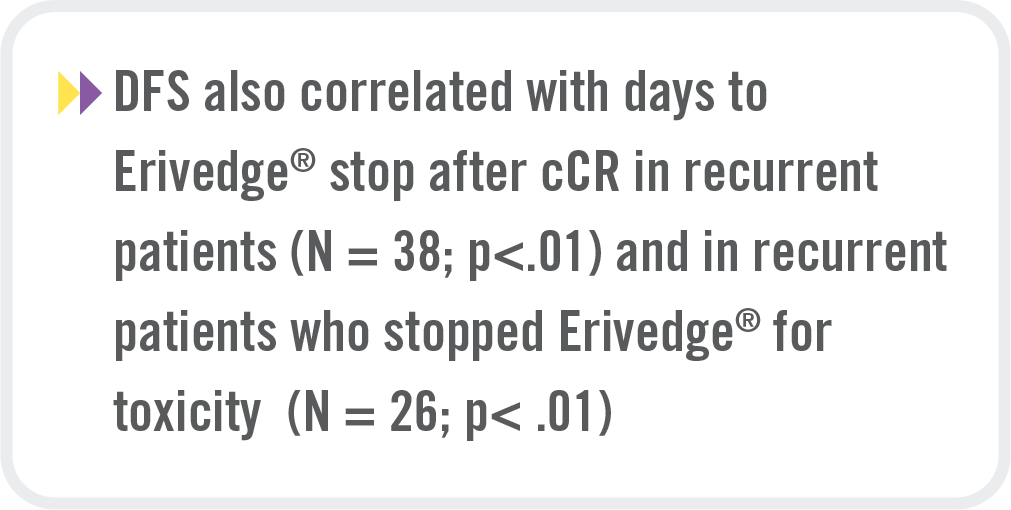
Correlation between DFS and treatment duration |
|
DFS was longer when Erivedge® was maintained for >2 months after cCR (mDFS > 2 months: 470 days vs. mDFS ≤ 2 months: 175 days, p = .01) |
|
|
Please see full prescribing information for additional important safety information |
|
| M-XX-00018375 |